A Genetic Approach to the Study of Simple Cognitive Abilities in Animals


Опубликована Сен. 26, 2014
Последнее обновление статьи Сен. 14, 2022
Abstract
One of the urgent problems in cognitive studies and in neurobiology as a whole is to delineate the impact of genetic factors in the variability of animal cognitive abilities. The concept of animal cognition is frequently used in a broad sense to include all phenomena with behavioral manifestations of neural plasticity. The variable phenomena related to animal cognition could be subdivided into two main categories, although only the first of them will be analyzed in this paper. The first category is represented by “basal” cognitive abilities, which embrace spatial cognitive behavior and elementary reasoning (simple logic task solutions). The second one mainly concerns more complex cognitive abilities including tool manufacturing in new situations, generalization up to the level of pre-verbal concept formation, symbolization, etc. The genetic influence on cognitive processes in animals may be investigated only in the domain of basal cognitive abilities. Studies are conducted mainly in rodents using different experimental models. However, performance has been typically compared in individual animals which are presumably different in genotype (e.g., selected animals and mutants). In this paper, a short review of such studies is followed by the description of original data on rodents’ elementary logic task solutions obtained in our experiments. The experiments used unique genetic models and an extrapolation task that addresses a relatively simple basal cognitive trait. Our experiment on selection for high extrapolation ability scores was performed for the first time, and inter-strain differences which emerged in these animals are described.
Ключевые слова
Rodents, extrapolation ability, cognitive tests, physiological and genetic mechanisms, selection for cognitive trait, cognitive abilities, animal reasoning, genetic models, robertsonian translocations, selected lines
1. Introduction
General Issue
In the early days of animal behavior studies, the cognitive abilities of various animal species were regarded as equal to their learning capacities, although Charles Darwin pointed out that animal reasoning is a distinct category of adaptive behavioral acts. During the 20th century, experimental evidence accumulated which confirmed this point with the main impact derived from experiments on ape logic problem solving capacities (Kohler, 1921; Ladygina-Kots, 2002; Firsov, 2010; Firsov & Chizhenkov, 2003). Edward Tolmans (1932, 1948) and Leonid Krushinskys (1990, 2009) experimental impacts and their general conclusions made it apparent that various types of cognitive behavior are also represented in the behavior of non-primate vertebrates (Bagotskaya, Smirnova, & Zorina, 2012; Olton, 1977; Olton & Samuelson, 1976; Olton et al., 1992; Meek, Church, & Olton, 1984/2013; Koehler, 1956; Wasserman & Zentall, 2006; Zorina & Smirnova, 2013; Zorina & Obozova, 2012).
The body of experimental results obtained in the field of animal reasoning has made it possible to claim that the existence of these abilities is a real phenomenon, distinct from behavioral acts based on habit acquisition (i. e., on learning per se)1 (Darwin’s views on the evolutionary role of animal behavior and on animal reasoning offered ideological support for Leonid Krushinsky, who initiated his novel experiments in the USSR (starting with extrapolation task experiments) at a time when it was almost impossible to deviate from Pavlovian conditioning theory (especially after the infamous Pavlovian meeting of two academies in 1950). At the end of the 1960s, Krushinsky defined elementary animal reasoning as the ability of an animal to apprehend the empirical laws which act in the external world and which determine different types of connections between objects and events, and the animal’s ability to program its adaptive behavior accord-ing to these laws. The terms “animal cognition” and “animal cognitive abilities” appeared later and embrace a wider range of phenomena including instrumental and classical conditioning, perception, attention and habituation) (Zorina & Poletaeva, 2001 /2011).

According to contemporary views acquired both in cognitive science and in neurobiology, animal cognitive abilities include phenomena which differ according to the level of complexity (Zorina & Smirnova, 2013; Poletaeva & Zorina (eds), 2013; Wasserman & Zentall, 2006; Reznikova, 2007). Animal cognitive abilities embrace at least two major categories of phenomena. The first one consists of basal, universal forms, which are actually inherent to all vertebrates. They include spatial behavior and memory, elementary logic tasks solutions including extrapolation ability, which will be described below (Krushinsky, 1990, 2009) and generalization capacity of a low level (Wasserman & Zentall, 2006). The second category includes capacities inherent to several groups of higher vertebrates (primates, dolphins, passerinae birds and parrots). In addition to possessing basal cognitive functions, these animals are able to demonstrate much more complicated forms of cognition, including mental functions ranging from complicated logic task solutions up to tool manufacturing in new situations (Firsov, 2010; Firsov & Chizhenkov, 2003; Shumaker, Walkup, & Beck, 2011), as well as generalization up to the level of pre-verbal concept-formation, symbolization and some types of logic inference (Zorina & Smirnova, 2013).
It is not possible to compare animal cognition both for basal cognitive abilities and mental operations of a higher order in the framework of this paper. The present review will deal mainly with the analysis of spatial learning and memory, as well as elementary logic task (extrapolation) solutions. A comparison of cognitive behavior of different degrees of complexity within the same species could also be instructive. It is also evident that genetic studies of animal cognition are currently possible only in the range of basal cognitive abilities. The respective models are represented mainly by rodent strains and stocks (predominantly rats and mice). It should be mentioned that data on differences in rodent strains selected for high and low learning abilities (i.e., RLA vs. RHA, Trayon maze bright vs. Trayon maze dull rats) are not covered by this review, although many authors consider them to be the main genetic models for cognitive behavior study.
Animal Reasoning: Extrapolation Ability. Comparison of Extrapolation Ability in Wild and Domestic Mammals
Leonid Krushinsky (1911-1984) belonged to the Russian school of experimental biology, led by prominent scientist Nikolay Koltzov. Krushinsky s interest in animal behavior combined successfully with his experimental skills and vast experience studying dog behavior and biology. At the same time, he was an ardent naturalist who made a lot of interesting discoveries while watching wildlife in Russia’s Taiga forest. One of his observations led him to the discovery of a new animal reasoning paradigm — the extrapolation task.
As the story goes, Krushinsky’s dog discovered a quail which ran away quickly in a straight trajectory and disappeared inside a line of bushes which was surrounded by open space. Instead of trying to penetrate the thick bush, the dog slowly went around it and waited for the prey where it would be expected to appear presuming its trajectory was still straight. This episode (and several ones of the same sort) was the starting point for Krushinsky to suggest that an animal could not only learn, but could also possess the capacity to understand the relationships which connect events and objects in the environmental world and to use this understanding for adaptive behavior. The behavioral reactions which occur in situations when no previous training could help — that is, when identical previous experiences (i.e., learning) do not exist — served for Krushinsky as the essential empirical ground for the new cognitive test which was introduced in his laboratory practice in the mid-1950s. That was the test for extrapolation (see Fig. 1).
Krushinsky s test for extrapolation consisted of the following components. A hungry animal sees the food bait via a vertical slit in a rather large opaque screen (large enough for the animal to begin eating through the slit), and the animal starts to eat. After a few seconds, the bait moves aside and disappears from the animals view. The correct solution of the problem is for the animal to move in the direction in which the food disappears, extrapolating the foods future position (in which it becomes available again). The solution requires that the animal: i) masters the law (rule) of object permanence: the object, which was seen but has disappeared, still exists and ii) is able to understand laws of motion: that if an object moves straight, it will be found in a more or less predictable place.
Vertebrate animals of many taxonomic groups have been studied using this test, which made it possible for researchers to build the variant of scala naturae, ranking animal species according to their success in the extrapolation task. This range coincides mainly with the degree of species’ brain complexity (Krushinsky, 1990, 2009; Zorina & Poletaeva, 2001 /2011).
Two more elementary logic tasks were introduced into laboratory practice by Krushinsky (1990,2009). They were:
- A test which evaluates the capacity of an animal to understand that the bait (which possesses a certain volume) could be hidden only in a voluminous (3-D) geometrical object, such as a cube or pyramid, and could not be hidden in the flat object of the respective geometrical form, such as a cardboard square or triangle, respectively. At the start of the experiment, an animal perceives both figures which stand upright in front of it. The figures start to rotate slowly, thus demonstrating their flatness and/or voluminousity.
- The so-called Revecz-Krushinsky test, in which animals have to determine the rule of hidden object displacement. The subject is presented with a row of small upturned cylinders. At each task presentation, the bait is hidden under one of them. An animal starts the food search and upturns several of these cylinders until it finally finds the bait. At each successive presentation, the bait is placed under a nearby cylinder. Thus, the place of the hidden bait changes systemically from trial to trial along the row of caches (Pleskacheva & Zorina, 2012).
Both tests are much more difficult for many animals than the test for extrapolation. Several species which master the extrapolation test were not able to solve the more complex tests, including dogs. Thus, dimensionality and Revezs-Krushinsky tests address the second category of cognitive abilities (not basal, but complicated) and their results will be analyzed elsewhere.
2. Genetic Approach to Animal Cognition: Progress and Difficulties
The main difficulty in genetic research of animal behavior is the high complexity of the respective traits. Although the laboratory mouse is rather well investigated in both physiology and genetics (e.g., Goldowitz et al. (eds), 1992; Crabbe et al., 1999; Wahlsten, 2011), the search for the genetic base of many behavioral traits is still problematic. Several hundred monogenic and chromosomal mutations in mice have been described, which have been shown to influence brain morphology and behavior (e.g., Boake et al., 2002; Williams & Mulligan, 2011; Wahlsten, 2011). Purposive connections — beyond simple correlations — were established between gene mutation and changes in behavior (Schwegler et al., 1990, 1991). However, researchers face difficulties of the highest degree when the analysis of cognitive abilities is performed (Lipp et al., 1989, 1996; Schwegler et al., 1990, 1991; Upchurch & Wehner, 1989; Wehner et al., 1997; Steinberger et al., 2003).
Rodents, which are most appropriate for genetic and physiological traits studies, proved to be “critical” with respect to extrapolation ability scores. This ability is weakly developed in laboratory rats and mice. In the 1960s and early 1970s, two unique experiments were performed in Krushinsky’s laboratory. Two “parallel” pairs of mammalian forms (wild and domesticated) were tested for extrapolation ability: wild vs. domesticated foxes (the latter were silver foxes from fur farms) and wild vs. laboratory rats. Wild and domesticated foxes solved the extrapolation task in a significantly non-random way, with wild foxes significantly superior to domesticated ones in this test2 (Domesticated foxes used in these experiments were animals obtained in the course of a well-known unique domestication experiment, which started at the Novosibirsk Institute of Cytology and Genetics (USSR Academy of Sciences) in the early 1960s (see Trut, 1999)). Further details of extrapolation ability studies using rats and mice of different genotypes will be given below.
3. Basal Cognitive Abilities in Animals. Spatial (Mental) Mapping in Animals and Genetic Approaches in Search of Mechanisms
Animal capacity for spatial learning and memory, extensively investigated for more than 30 years, belongs to the category of basal cognitive abilities. This type of cognitive ability requires the formation of mental representations of spatial environmental characteristics. The investigation of “spatial map” in laboratory rats was started by Edward Tolman (1886-1959) and is studied in the paradigm of orientation skills acquisition using radial and Morris water mazes. The concept of a given environments spatial organization, once developed, could be used by an animal in the future, in that an animal is able to utilize a spatial memory engramm3 (The prominent Russian zoologist Valentin Pazhetnov, a brilliant specialist in brown bear behavior, made a detailed description of how the wild bear thoroughly mastered the space of his individual area using shortcuts and navigating through previously unused parts of the wood-land territory (Pazhetnov, 1990)) . The spatial memory is not a uniform phenomenon; it may be operant memory (i.e., the memory traces used during this rather short time interval) or working memory (the traces which are needed to solve the problem with the given constellation of objects and events). It had been obvious from the beginning of such studies that this conceptual framework is not compatible with a “stimulus-response” (SR) paradigm. The experiments using these techniques made it necessary to revive Tolmans ideas, which were formulated very much ahead of his time.

The History of the “Mental Map” Idea
Tolman was influenced by the ideas of the Gestalt psychologists, although he belonged to the Behaviorism scientific school by his prime interests and chosen methodology. Tolman used behavioral methods to study and understand mental processes, an idea which strongly opposed the main behavioristic paradigm. Tolman experimented with rats who learned to navigate complicated mazes, which served as the basis for his “cognitive map” hypothesis. This hypothesis was supported by numerous successors in spatial learning and spatial orientation experiments several decades after Tolmans initial experiments (Olton, 1992). In his studies of learning in rats, Tolman sought to demonstrate that animals could learn facts about the world which they could subsequently use in a flexible manner, rather than simply learning automatic responses that were triggered by environmental stimuli. In his famous paper “Cognitive Maps in Rats and Men” (1948), he claimed that he belongs not to the school of scientists who stress the prime importance of the stimulus-response principle, but to the school of so-called field theorists. He wrote: “...we believe that in the course of learning something like a field map of the environment (italic ours) gets established in the rats brain. We agree with the other school that the rat, in running a maze, is exposed to stimuli and is finally led as a result of these stimuli to the responses, which actually occur. We feel, however, that the intervening brain processes are more complicated, more patterned and often, pragmatically speaking, more autonomous than do the stimulus-response psychologists. Although we admit that the rat is bombarded by stimuli, we hold that his nervous system is surprisingly selective as to which of these stimuli it will let in at any given time.” This is the idea of the “mental map” formation.
Around the same time period, Ivan Beritov (also known as Ivan Beritashvili) made a series of experiments with dogs on their orientation in space highlighting the involvement of recent memory traces. The interpretation of these results was also based on the concept of respective “internal representations” in these animals (Beritov, 1967).
Radial and Water Maze Era
The first experimental data from the “new wave” of interest in the spatial map problem developed from hippocampus studies, which indicated that hippocampal lesions resulted in changes which looked more or less confusing when authors tried to explain them in the framework of the SR paradigm (e.g., Olton, 1972). The first test which aimed to evaluate animals’ spatial learning and specifically the importance of working and reference memories was the radial maze test (Olton, 1977). In the first experiments and in hundreds of others which followed, the navigation of an animal was proven to “work” in the space created in a lab room by eight or more maze alleys (arms) extending from a central area. An animal (usually a rat or mouse, although some other species as well) uses beacon cues from outside of the maze to find the respective arm in which the food is located. The “spatial” but not SR mode of animal orientation, both in the “classic” radial maze paradigm and in its different versions, demonstrated the real existence of specific spatial knowledge and memory (Olton, 1992; Olton et al., 1992; leather, Packard, Smith, Ellis- Behnke, & Bazan, 2005; Wahlsten, Cooper & Crabbe, 2005; Lerch et al., 2011). One of the early works in this field was that of Olga Buresova, who demonstrated that rats which are overtrained in the 12-arm radial maze show considerable transfer of the habit to a maze consisting of 12 parallel alleys entered from a common choice area. When isolated maze channels equipped with one-way doors on both ends are randomly scattered over an enclosed area of 2 m2, the rats were able to visit them sequentially, even when they encountered this particular configuration for the first time (Buresova, 1980). The role of the hippocampus in this type of behavior was confirmed in subsequent studies (Morris, 1984; Buzsaki et al., 1990; Goh & Manahan-Vaughan, 2013).
The water maze test was introduced by Richard Morris several years after Olton’s radial maze test (Morris, 1981), and both tests quickly became popular. The experiment is usually performed in the following way. The experimenter place an animal into a circular tank filled with water. The animal is motivated to avoid the unpleasant water milieu. In search of a plausible exit, it swims around the whole area and occasionally finds the safe platform, hidden under the water surface (water in the tank is made opaque in order to prevent searching the platform visually). The special visual cues on the walls around the water tank serve as visual beacons, which provide the animal orientation in this space. During further task presentations, the animal is released each time from a different point of the tank’s periphery. The spatial learning of an animal is assessed by shortening the platform search time. It is also possible to use the “cued” (i.e., operant) version of the same test in which the platform is made visible and can be marked by a special cue. It was also demonstrated that the success of finding the hidden platform in the “spatial” version of the test depended on hippocampal (and other structures) function. Thus, this test permitted a comparison of animal performance in the “cued” (operant) and “spatial” (cognitive) test versions in order to reveal the specificity of the physiological mechanisms involved in their performance (Wenk et al., 1987; Sara, Devauges, Biegon, & Blizard, 1994; Pleskacheva et al., 2002; Grootendorst, Enthoven, Dalm, de Kloet, & Oitzl, 2004; Lerch et al., 2011; Dragoi & Tonegawa, 2014;
Miyoshi et al., 2012; Sultana et al., 2013; Arp et al., 2014). The plausible gene expression differences were analyzed as well (Paratore et al., 2006; Steinberger et al., 2003, among others). Using the technique of early gene expression in the mouse brain, it was demonstrated that distinct and simultaneously working plasticity mechanisms are active during different phases of the Morris water maze training (Laeremans et al., 2014). The differential involvement of rostral and caudal hippocampal areas in spatial “knowledge” formation was demonstrated as well (Kuptsov, Pleskacheva, Voronkov, Lipp, & Anokhin, 2006, 2011). Hippocampal place cells, discovered 40 years ago, have been extensively studied, and it was demonstrated that they provide an exquisitely detailed representation of an animal’s current location and heading. The key properties of the major categories of spatial cells — place cells, head direction cells, grid cells and boundary cells — were specified (Hartley, Lever, Burgess, & O’Keefe, 2014).
Mossy Fibers Projection Size and Spatial Competence
A series of extensive studies began in the late 1980s which demonstrated that rodents’ performance success in the radial maze and Morris water maze tests correlated with the size (area or volume) of infra- and intrapyrami- dal mossy fiber (iiP-MF) projections in hippocampal CA3 pyramidal neurons, while no genetic variation was found for the suprapyramidal layer MF projection field (Schwegler, Crusio, & Brust, 1990, 1991; Crusio, Schwegler, & Brust, 1993). This type of correlation reflected the real participation of a definite hippocampal circuit in spatial behavior and was shown for several mouse and rat strains differing in spatial navigation capacities. Moreover, the early postnatal thyroxin treatment of DBA/2 mice (chosen because of scant iip-MF projections and poor radial maze learning) induced the increase in the volume of iiP-MF and the decrease of errors in the radial maze test. Parallels were also found in Morris water maze scores, the size of iip- MF and ecological specialization of two wild vole species, namely Clethrionomys glareolus and Microtus oeconomus (Pleskacheva et al., 2000). The differences in spatial memory and iip-MF scores corresponded to the ecological lifestyle of these two species and were in line with previous observations on the role of the iip-MF.
The performance in spatial tests by mice and rats of different genotypes had been analyzed since early descriptions of these tests in numerous works (e.g., Ammassari-Teule & Carpioli, 1985; Nguyen, Abel, Kandel, & Bourtchouladze, 2000; Yoshida, Goto, & Watanabe, 2001; Sunyer et al., 2008; Patil, Schlick, Höger, & Lubec, 2010; Gök^ek-Sara^, Karakurt, Adah, & Jakubowska-Dogru, 2012; Matsuo et al., 2010; de Bruin et al., 2006). It is not possible to make even a short analysis of the respective publications as the field is extremely broad. The roles of several signalling cascades in different brain areas (hippocampus, prefrontal and enthorhinal cortical and striatal areas) were demonstrated (Becker, Walker, & Olton, 1980; Pang, Williams, Egeth, & Olton, 1993; Lerch et al., 2011; leather et al., 2011; Miyoshi et al., 2012). Genome wide association studies (GWAS) and quantitative trait loci (QTL) techniques were successfully used to demonstrate that different sets
of brain genes were expressed during Morris water maze performance in comparison to the situation of passive avoidance learning (Steinberger et al., 2003; Wahlsten et al., 2005; Paratore et al., 2006). The chromosomal regions which non-randomly participate both in spatial learning success and in the accuracy of performance were also mapped (Ruiz-Opazo & Tonkiss, 2006; Herrera et al., 2013).
The numerous knockouts (KOs) of genes participating in brain function are accompanied by the impairment of spatial tests performance (e.g., Sarnyai et al., 2000; Wang et al., 2004; Duclot, Jacquet, Gongora, & Marice, 2010; Wincott et al., 2014, etc.) or by improvement in these behaviors (e.g., Hussaini, Kempadoo, Thuault, Siegelbaum, & Kandel, 2011; Yadav et al., 2013; Terunuma et al., 2014). The latter cases are of special interest as these data could point to the most important links in the chain of neuronal events which underlie the cognitive behavioral act from the domain of spatial orientation and behavior.
No special efforts have been made to analyze the performance of animals in spatial tasks using genetic approaches other than mutation analysis — namely, no selection experiments with further analysis of behavior in hybrids were done. The hybrid F2 mice from a cross between C57BL/6J and 129sv mice was also used in order to make the frame of reference as numerous KOs were created using this genetic background, and it was shown that these mice outperformed mice of both parental strains in the Morris water maze test (de Bruin et al., 2006). Usually, the hybrids of different rat or mouse strains were used in the radial maze and Morris test studies for the purpose of examining different drug treatments (e.g., Hasenohrl et al., 1999), as Fl hybrids’ performance is usually much more uniform than that of inbred-strain animals. The use of F1 in these studies makes the interpretation of drug effects more reliable. The selection experiment, especially using rats as subjects, is a time-consuming and costly enterprise. This point could easily be regarded as a reason against such types of study, although the small (and probably not consistent) range of individual differences demonstrated by rats and mice in these tests could be another reason. The second may not be true as mouse strains (e.g., DBA/2 and C57 BL) showed consistent and reliable Morris test differences (Voikar, Polus, Vasar, & Rauvala, 2003).4 (During the past 20 years, the radial maze and Morris water maze tests were extensively used in pharmacy and pharmacology research. This indicates that the techniques are actually of rather high practical importance. Thus the methodology first introduced for fundamental animal behavior research proved to be important for practical needs as well.)
Overall, spatial cognitive abilities, at least in rodents, have been studied extensively. The main results of physiological and genetic investigations of this function reveal that these processes, as well as instrumental and classical conditioning, represent those behavioral phenomena in which neural-behavioral plasticity was manifested. At the same time, spatial orientation paradigms imply the functioning of mechanisms which are different from those involved in other forms of learning.
4. Basal Cognition in Animals. Elementary Logic Task Solutions.
Is it Possible to Find out How Genotype Influences This Behavior?
The short history of extrapolation ability studies, initiated in Krushinsky s laboratory, were presented in the Introduction in order to describe the main features of the experimental approach used in our study. Even after the first experiments on extrapolation ability in dogs, cats, crows and other species, it became clear that individual differences exist in this task performance. Of course, the idea emerged that developmental biases and genotype may be factors underlying this variability.
Start of Krushinsky’s Studies of Animal Extrapolation Ability Using Genetic Approaches
The role of genotype in extrapolation test performance was first studied in comparative experiments using wild and domesticated animals. Two pairs of forms (wild and domesticated foxes and rats), presumably different in genotype, were compared for their extrapolation ability at the first task presentation, when no previous experience of this task solution (i.e., learning) could influence the results (for a review, see Poletaeva, Popova, & Romanova, 1993). The scores of multiple presentations of the extrapolation test were also analyzed.
The data on experiments with rats of different genotypes are presented in Figure 3. As in the case with foxes, extrapolation performance of the wild brown rats was significantly higher than that of laboratory rats, although unlike domesticated foxes the laboratory rats performed poorly in this task (their scores were not different from random choice level). The Fl hybrids between wild brown rats and rats of a laboratory strain (audiogenic seizure prone strain — KM) were obtained. These animals performed excellently in the extrapolation task, although the tiresome procedure of handling them was applied to each pup during the first two to four weeks of life, in order to tame them as a prerequisite for testing them in the extrapolation box. If not handled, the hybrid rats (as well as wild Norway rats) developed the extreme forms of anxiety and “fearful” aggression. Hybrid rats of the initial Fl population did not display an increased fear reaction, and those animals of further generations which were chosen for breeding to obtain animals of each next generation were not fearful either. The summarized extrapolation scores of F2 - F4 rats were not higher than those of Fl (i.e., hybrids between wild and laboratory rats), and these animals in their majority were extremely fearful in the situation of the extrapolation testing box. Thus, animals from F2 - F4 generations were fearful despite intense handling during adolescence, similar to that of Fl. This increased fear was an obstacle to obtaining data when the extrapolation test was used, as these animals did not approach the food, although they were very hungry. After the failure of the first selection experiment, similar selection attempts were made two more times with the same results. The further breeding of rats was stopped in all cases, as it was not possible to test extrapolation ability in animals displaying intense fear in the experimental box. The plausible explanation of increased fear in the progeny of rats which were selected for breeding because of their fearfulness could be the close “causal” connection between the genetic basis of this cognitive trait (high extrapolation scores) and those brain mechanisms which provide fear responses.

Extrapolation Test Details in Experiments with Mice
In further experiments in the area of behavior genetics of extrapolation ability, laboratory mice were used. The experimental device for testing extrapolation ability was different from the “classic” screen test used for larger animals (see Fig.l). The reason for this change was to make it possible not to remove an animal from the box after each test presentation, thus avoiding the unnecessary stressful stimulation. The device was the opaque open box which contained two reward chambers and the central feeding site (see Fig. 4). At these locations, the mouse could reach a small food cup containing milk through holes 10 mm in diameter. Two identical cups were mounted on the bar in front of the wall outside of the box and could be slid manually to the lateral feeding sites. One of these cups could be moved to the right, and the other to the left of the central feeding site. Mice were food and water deprived for 15 to 16 hours. On the test day, each individual mouse was placed into the box. The animal started to drink milk from one of the cups via the central opening (feeding site). After three to five seconds of drinking, the cup was moved slowly to the right or to the left. The mouse could follow this displacement for one to two centimetres of the trajectory and then the food cup disappeared from its view. The second cup (also containing milk) was moved in the opposite direction, remaining invisible to the animal. This was performed to balance the odor cues from both sides of the box. The cup from which the animal started to drink moved to and stopped in front of the respective side opening. The choice of the feeding location (either indicated by perceived movement of the food cup or chosen by chance) was registered as the correct task solution while the cases when an animal approached to opposite side opening was qualified as an incorrect solution. If no approach was performed for 120 seconds, it qualified as a “zero” solution. The experimental session included six trials. The data were presented as the two separate scores, with proportions of correct task solutions from the total number of them both for the first task presentation and for the six presentations in sum.
5. Cognitive Abilities in Mouse Extrapolation Ability in Mice with Chromosomal Mutations. Elevated Reasoning Ability in Extrapolation Test
Using Mice with Chromosomal Rearrangements (Anomalies)
As mentioned above, mice of inbred strains (CBA, DBA/2, C57Br, A/Не, BALB/c, 101/HY) solved the extrapolation test in most cases by chance, the proportion of correct choices rarely rising significantly above the chance level, although some exception were discovered as well. Experiments with numerous mice of different inbred strains show that in C57BL/6J and BALB/c mice, this score could be significantly above the chance level in several definite samples of animals, although no such prevalence of correct choices were noted in the CBA/Lac/Sto strain (these mice were also biased to alternate the direction of movement from the central opening to side holes in a strict left-right-left-right etc order). The incentive for analyzing extrapolation ability in mice was the search for the genetic group(s) of laboratory mice which reveal extrapolation ability in reliable and significantly non-random levels. Following this idea, we tested mice from the collection in the Embryology Department of Leningrad Institute for Experimental Medicine (IEM). Andrey Dyban, the head of this department, and Vladislav Baranov provided a large number of mice which had various chromosomal rearrangements.

Extrapolation Ability in Mice with Partial Trisomies for Autosoms
The specified breeding scheme of mice-carriers of reciprocal5 (Reciprocal chromosomal translocation is a chromosomal rearrange-ment which implies that two non-homologous chromosomes interchange their two fragments. During this process, at least two chromatides breaks take place) (not robertsonian) translocation T43 (16, 17) H and mice with robertsonian translocation (fusion) Rb (16, 17) 6Bnr was performed. In the progeny of such crossing, a small number of progeny carried two normal chromosomes N 17 and the excessive portion of chromosome 17, which was translocated to chromosome 16. Thus, these mice were partial triosomics for the fragment of chromosome 17. The extrapolation test was given to the group of mice with T43 (16,17), in which seven animals were shown to have the partial trisomy for chromosome 17.

The respective data are presented in Fig. 5. Mice which carried the T43H in a homozygous state revealed a high percentage of correct choices in the extrapolation task, and the presence of additional fragment of chromosome 17 in partial trisomic individuals was not accompanied by a decrease in task solution success.
It is noteworthy that these animals demonstrated very high scores for the first task presentation. In separate experiments, it was demonstrated that the locomotion level in these trisomic mice (number of squares crossed in the “open field” test) was not different from that of mice with normal chromosome numbers and identical genetic backgrounds.
At the same time, the pattern of extrapolation success scores was different for the similar groups of mice carrying another reciprocal translocation, T6 (14,15) Ca. Karyotype investigations of animals with this translocation also permitted the discovery of several animals which carried the small additional “marker” chromosome, T6, composed from small portions of chromosomes 14 and 15. It was another case of partial trisomy, in this case for portions of chromosomes 14 and 15. In these groups of mice, the levels of successful solutions of the extrapolation task were not different from the 50% chance level (see Fig. 4). It was also demonstrated that in mice with this type of trisomy, their learning capacity was impaired. The latter fact was not surprising as it was widely known that autosomal trisomy in humans is highly deleterious, causing severe mental retardation (Down Syndrome involves trisomy 21). Later it was demonstrated that mice-trisomics for the chromosomal portion, carrying genes analogous to human chromosome 21, performed the cognitive Morris test at low levels (Sago et al., 1998). Meanwhile, it was rather unexpected that in the case of partial trisomy for chromosome 17 fragment, no impairment of extrapolation ability occurred. The correct solution of the extrapolation task involves the trait of high complexity with probable adaptive value, and it may be inferred that partial trisomy for parts of different autosomes resulted in different consequences for extrapolation task success.
Robertsonian Translocation (Fusion of Chromosomes 8 and 17)
Mouse karyotypes consist of 19 autosome pairs and a pair of sex chromosomes (XX or XY) (Committee on Standardized Genetic Nomenclature for Mice, 1963) . All murine chromosomes are of the acrocentric type, which is the prerequisite for special types of chromosomal fusions which do not affect the cell and organism viability. This type of chromosomal rearrangement is known as robertsonian translocations6 (A robertsonian translocation (centric or, rarely, tandem fusion) involves two chromosomes of acrocentric type. During a robertsonian translocation, the participating chromosomes break at their centromeres and the long arms fuse to form a single metacentric or sub-metacentric chromosome. These type of translocations are found rather frequently in wild rodent populations. In Mus musculus and Elobius sp., the local isolated wild populations were found with individuals homozygous for several robertsonian translocations (Bakloushinskaya et al., 2010; Gropp etal., 1982)). A minimal loss of genetic material occurs as the result of such fusion, and mice-carriers of them are viable and fertile (Baranov, 1980).

A series of experiments on extrapolation ability were performed using mice with the fusion of chromosomes 8 and 17, which demonstrated their behavioral peculiarities.
Obviously, the extrapolation ability comparison in mice with different genotypes (and karyotypes) was of prime interest in our studies, as the successful solution of this test could serve as an indicator of reasoning ability in these animals. This test was the “central” one in these experiments and values of correct solution success were then compared with data on learning and several other behavioral indices. A detailed description of these experiments are presented in Poletaeva and Romanova (2013). The extrapolation test was initially presented to mice with the following robert- sonial translocations (RT): Rb (8,17) llem, Rb (5,19) lWh, Rb (6,15) lAld, Rb (9,14) 6Bnr, and Rb (16,17) 7Bnr. With the exception of Rb (8,17) llem mice, all carriers of other RTs solve the task at a level not significantly different from the 50% chance level. Many different groups of mice with Rb (8, 17) llem demonstrated task solutions levels which exceeded the random one with different degree of significance (Poletaeva & Romanova, 2013).
This initial finding — the non-random extrapolation task solution by mice with the fusion of two definite chromosomes (8 and 17) — induced the next series of experiments. One of the causes of the elevated extrapolation ability in mice with Rb (8, 17) llem could be the plausible differences in the genetic backgrounds of the mouse population in which this RT was found. They were C57BL related mice, although not inbred, but “mixed” with the genotypes of other, unidentified strains. The necessity to analyze the plausible influence of this factor induced us to breed new strains, in which mice-carriers Rb (8,17) llem possessed two different genetic backgrounds (C57BL/6 and CBA). Thus, the objective was to analyze the extrapolation performance of four strains which differed pair-wise either by genetic background or by karyotype (normal karyotype strains CBA and C57BL/6J, later CBAN and BLN) and new inbred strains CBARb and BLRb, which carried the Rb (8,17) llem. The latter pair was bred by brother-sister matings after a series of back-crosses of RT carriers to respective inbred mice.
The results of the extrapolation tests in mice of these four strains (see Fig. 6) demonstrated the following: 1) mice-carriers of Rb (8, 17) llem with both genetic backgrounds demonstrated the presence of extrapolation ability, and the proportion of their correct solutions was non-randomly above the chance level; 2) the performance of CBARb mice was not as successful as that of BLRb. These data mean that the impact of genetic background was not crucial in the determination of extrapolation ability, and that the presence of fused chromosomes 8 and 17 induced the increased ability to solve the elementary reasoning task.
Another factor which could be the cause of increased extrapolation ability in Rb (8, 17) llem carriers was the plausible fixation of beneficial alleles fixed in the “double” (fused) chromosomes; in case of RT, the crossover percentages was shown to be low (Gropp et al., 1982). Fixation of those alleles from chromosomes 8 and 17, which were beneficial for cognitive behavior and were present in the RT population, could occur. These “beneficial” alleles could be the cause of the elevated extrapolation ability in mice with Rb (8, 17) llem. The same fusion of these chromosomes may be found in other mouse populations. If these mice are also superior to other groups in their extrapolation ability, that would mean that it is the fusion of these chromosomes per se which determined the increased ability for extrapolation. The small sample of mice with the fusion of chromosomes 8 and 17 which occurred independently in the population of wild mice in Sicily was studied. The percentage of correct choices in these mice (both at the first task presentation and in sum for presentations 1 through 6) was highly significantly above the chance level (see Fig. 6). Summarizing these data, it is possible to claim that the fusion of these chromosomes (8 and 17) was the factor which induced some changes in the CNS function and that these changes were beneficial for the elementary reasoning task solution success.
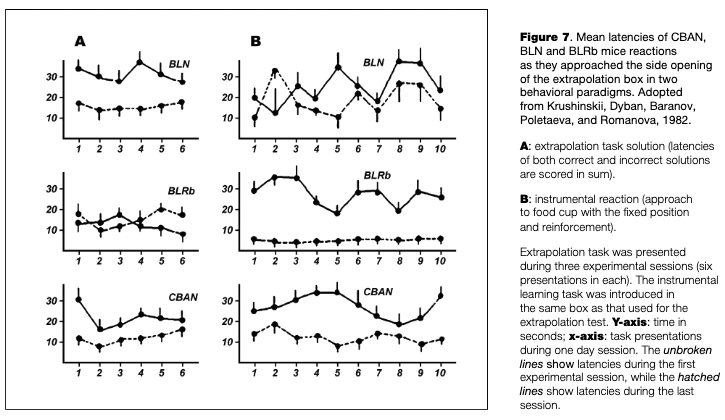
The behavior of Rb (8,17) Hem carriers was compared to that of mice with normal karyotypes in the paradigm of instrumental learning. In this test, the approach to one of the side openings of the extrapolation box was reinforced, with subsequent analysis of trials to criterion and subsequent reversals rates.
Fig. 7 shows the latencies of skill acquisition in mice of three groups during the first and last days of training (B). The shortening of these reaction times, as the indicator of learning, was much more prominent in mice with the RT in comparison to CBA and C57BL mice. At the left side of Fig. 7, the latencies for extrapolation task solutions in the same animal samples are presented for comparison (A). It may be seen that the movements of mice with RT was quicker than in the other two groups, although no shortening of this reaction occurred from the first to the last experimental days. These data signify that mice with RT solve the extrapolation task relying mainly of the information learned during the task presentation, while the other mice (BLN and CBAN) succeed mainly by improving the motor component of the task.
Morris Water Maze Test in Mice with Rb(8,17) I lem
The small sample of female mice (n = 9 for each group) of the four strains mentioned above (CBAN, CBARb, BLN and BLRb) was tested using the Morris maze test of the Institute of Anatomy (University Zurich-Irchel, Zurich, Switzerland; see Leitinger et al., 1994). The extrapolation ability of these animals was in the range shown in Fig. 5, while the results of the spatial cognitive task were different. With the exception of the BLRb group, all mice tested acquired the habit of “discovering” the hidden platform successfully, their scores being in the range of the other strains tested previously. A three-factor ANOVA demonstrated the independent influence of the factors genotype (F(l,28) = 5.067, p = .0085) and RT (F(l,28) = 4.36, p = .012). The translocation effect was higher in the BL pair of strains than in CBA mouse groups. The reaction times of finding the platform in mice of the BLRb group were longer than in the other groups, although all of the animals learned this task. Only the RT factor (and not genotype) significantly influenced the time spent on the former platform quadrant by mice of these groups (LSD Fisher post hoc test, p - .031) . The possible explanation of these data was that the Morris test procedure induces more intense fear (and stress-related behavioral shifts) in mice with RT than in animals of the other groups. This in turn hampered their capacity for spatial orientation in the test. This explanation finds some support from the same experiment, as the significant influence of the RT factor was found for floating time and thigmotaxis scores. These two Morris test indices are usually regarded as the signs of anxiety and stress susceptibility, and thus the still-unexplained plausible connection between this mouse cognitive trait and anxiety performance emerged in this case as well (Leitinger et al., 1994).

6. Mice, selected for large and small relative brain weights. Differences in behavior and in extrapolation ability in particular.
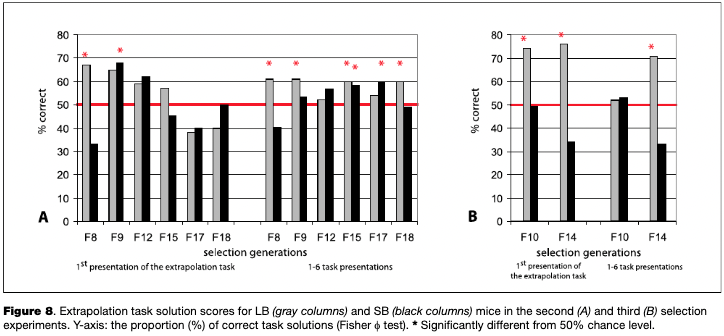
Brain weight is an important morphological index, which has been traditionally used for comparison of CNS development in different animal systematic groups, although relative brain weight (brain weight divided by body weight) is considered to be a more informative index (Kruska, 1975 and 2005; Rensch, 1956; Rehkämper et al., 1991). Various factors influence brain weight values within the same species, including genotype, ecology, environmental prenatal toxicity, and developmental biases (Henderson, 1973; Markina, Salimov, & Poletaeva, 2001). Three experiments in which two lines were selected for large and small relative brain weight (LB and SB lines) were performed in our laboratory.7 (The selection of mice for large and small relative brain weight was performed according to the following procedure. At the age of 1.5 months, half of the animals from a given litter (mice born to the same parents) were sacrificed and their brain and body weights were determined. If the scores for a given litter fell above (for LB) or below (for SB) the respective regression line (brain-body weight) which was created for the previous generation, mice from the other half of the given litter were used for further breeding (Poletaeva et al., 1993; Perepelkina et al., 2013)) In all three experiments, LB mice displayed more efficient learning and more successful extrapolation task performance, although not in all generations (see Fig. 8). At the same time, SB mice were more fearful and less stress resistant, as well more inclined to display stereotypic reactions. The replication of selection data is the requirement for selection experiments which have to demonstrate that the differences found were not the result of chance allelic association. Below, several experimental facts from the third selection experiment will be demonstrated. It should be mentioned that in all cases, the selection for LB and SB resulted in significant inter-line differences rather quickly — after four to six selection generations with the accompanying differences in behavior (Poletaeva et al., 1993; Perepelkina, Goli- brodo, Lilp, & Poletaeva, 2014).


Investigating differences in brain weight in mice of different strains usually revealed the influence of such factors as sex, body weight, age, as well as methodological details of brain tissue histological processing (see website of R.W. Williams http://www.nervenet.org/iscope/mbl 10.html).
It is also clear that genetic polymorphisms for brain weight differences in the population are due not to rare mutations but to different frequencies of the respective alleles. However, it is commonly accepted that the increase of brain size is a progressive evolutionary trend (Kruska et al., 2005) and the proofs were shown on the species level (Popova & Poletaeva, 1983; Poletaeva et al., 1993) 1. The QTL study of brain weight suggested several loci to exert non-random effect on this trait, being located on chromosomes 15,16 and 19 (Peirce, Chesler, Williams, & Lu, 2003).
At the level of F22 of the third brain weight selection experiment, the selection procedure was stopped and animals were bred further at random inside each of the lines during seven generations. The parents for the next generation (four males and six females) were chosen by chance and placed in larger cages (34 x 29 x 17 cm).
In F23-25 generations, the brain weight in LB and SB mice was not determined. In F25-28, the brain weight was measured, although the choice of breeding pairs for the next generation was still determined at random. In F25-28, the LB — SB brain weights differences were still highly significant (pciO’5 — 10’6).
LB and SB mice from F28 (the seventh generation without selection for brain weight) were compared for solution of another cognitive task — the “puzzle box” test.
“Puzzle Box” Test, Experiments with LB and SB Mice
The puzzle box test (for details see Ben-Abdallah et al., 2011; Perepelkina et al., 2014), is a modified version of the “Light- Dark” test, in which an animal is placed into the brightly lit part of a box and must find the way to escape into the darkness; the route to the dark compartment (goal box) was via an underpass (4x2x15 cm)8 (The entrance into the dark part of the box could remain without obstacles or be blocked by either wood shavings (“burrowing puzzle”) or by a T-shaped card-board-plastic plug (“plug puzzle”). There were eight stages of the test, presented during two experimental days. The test start-ed with simple stages 1 and 2 (the animal can freely enter the dark part of the box), while the next stages (3 through 5) already had a “cognitive” component— the underpass was filled with wood shavings to the lev-el of the box floor. Stages 6 and 7 followed, in which the underpass was blocked by the light plug, which the mouse could easily lift and put aside in order to penetrate the dark part of the box. At the final stage, the wood shaving heap (5 – 7 cm high) was placed along the whole wall with the under pass (for details see Ben-Abdallah et al., 2011).) (see Fig. 9).
A cognitive component of this task solution exists as an animal should comprehend that even if the entrance to the goal box was not seen, it still existed (object permanence rule). Thus, in order to succeed in this test, an animal has to understand this rule.
The mean time scores (latencies to enter the goal box) for a group of animals were used to measure the task solution success. The results of the puzzle box test, presented to 15 LB and 14 SB mice of F28, are shown in Fig. 10.
The latency of task solution (the time from a mouse being placed into the box until the moment when it penetrates the dark compartment) was the measure of task success. If a mouse did not enter the dark part of the box, a latency of 180 seconds was ascribed (for trials at stages 1 to 5 and 10). A latency of 240 seconds was the “deadline” for the most difficult stages, 6 and 7, when the light plug prevented the entrance of an animal into the goal box. The performance of LB mice at all stages of this test was more successful than that of SB animals. Mice tested in this experiment belonged to F28, when the selection was discontinued for seven generations. Previously, it was demonstrated that SB mice (in all three replications of brain weight selection experiments) were consistently more fearful than LB animals (see Perepelkina et al., 2014). Thus, they presumably would be more eager to escape into the safe dark compartment, while the less fearful LB mice were more successful in the puzzle-box test performance. As the solution of this test had a clear-cut cognitive component (i.e., an animal must understand the “object permanence” rule), the results obtained permit us to conclude that the cognitive abilities of LB mice are higher than those of SB animals.
7. Selection Experiment in Mouse Why not Total Success?
The selection of mice for high extrapolation ability was started on the basis of a genetically heterogeneous population. They were F2-F4 hybrids of crosses between strains selected for large and small relative brain weight, in turn derived from six inbred strains (Poletaeva et al., 1993). The criteria for selecting animals as parents for the next generation were: 1) correct task solution at the first extrapolation task presentation, five-to-six correct solutions out of six total task presentations, lack of hesitation in approaching food (no refusals, see above), no “zero” solutions (whereby no choice was made during 120 seconds). The lack of refusals and of “zero” solutions were indications of a low level of anxiety in a given animal in the situation of the extrapolation test. Thus, the selection program adopted in this experiment included two behavioral traits to be selected for: animals should demonstrate a high extrapolation ability and low anxiety during the testing procedure. Control mice (CoEX) originated from the same heterogeneous population and were bred at random.
During the first generations (F4-F9), mice of the selected line (EX) showed percentages of correct solutions which were significantly above the 50% chance level, while control mice scores were not different from the random level (Perepelkina et al., 2011). The pattern of inter-strain differences changed starting from F10, in which sex differences emerged and stayed in Fll and F12 (see Fig. 11). Moreover, in Fll the scores of EX mice were not significantly different from the 50% chance level. The CoEX mice proportions of correct choices were significantly above the chance level in Fll males and in F12 females.
Therefore, the selection of mouse strain for high scores of extrapolation task solutions could not be considered successful. This indicates that extrapolation ability should be regarded as the cumulative “positive” cooperative action of many factors. It is possible to suggest that the trait “high extrapolation success”, as one of an animals cognition manifestations, is determined by multiple genetic factors with non-additive interactions, as this trait could play a role in survival and participate in species fitness determination.
The cognitive ability in the form of extrapolation ability was not revealed in mice as the result of special selection for this trait, although the differences in the other test with definite “cognitive” components could be revealed between EX and CoEX mice.
This suggestion was confirmed by the results of the puzzle box test, introduced to EX and CoEX mice of the last selection generations. Fig. 11 demonstrates the proportions of animals from both genetic groups which were able to solve the most difficult stages of this test — that is, the ones which required the removal of the plug as the obstacle for entering into the “safe” dark compartment of the box. These proportions were larger for EX mice with a rather high level of significance.
The results of the puzzle box test could be interpreted as confirming the selection success for cognitive ability in mice, in spite of the fact that the response to selection for the trait of interest — extrapolation scores — was very weak.
The data from the hyponeophagia test could be one additional confirmation for this conclusion.
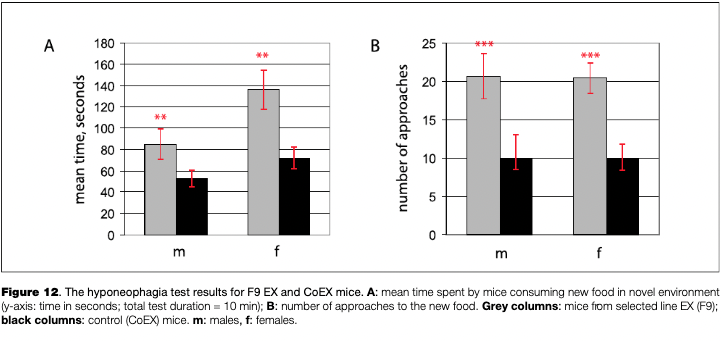
The hyponeophagia test aims to measure the reaction to novelty, when a food-motivated animal is placed into a novel environment with a new kind of food to consume (Dulawa & Hen, 2005). In this test, a mouse which has been food-deprived for 18 hours is placed for 10 minutes in a dimly lit circular plastic chamber (40 cm in diameter), and given a small portion of cheese (1.5 x 1.5 x 1.5 mm cubes). The time spent consuming food, the number of approaches to the food and the weight of the cheese eaten are estimated for each animal. Tested in several selection generations (F8-F11), the hyponeophagia test gave highly consistent results: EX mice spent more time eating, approached the food more frequently and in most cases ate more of the new food, compared to CoEX animals (see Fig. 12).
In rodents, the reaction to novelty is influenced by two factors: anxiety level and an inherent tendency to explore a new environment (Clinton, Stead, Miller, Watson, & Akil, 2011). The lower scores of this test in EX mice in comparison to CoEX mice could reflect differences in both the anxiety behavior and the attitude to novelty9 (The reaction to novelty was recently shown in four populations of great tits (Parus major), and it was demonstrated that it correlated with the scores for spatial orientation and depends on haplotype of D4DR, known to be associated with novelty reaction in humans (Korsten et al., 2010; Mueller et al., 2013)) . The latter factor deserves special analysis.
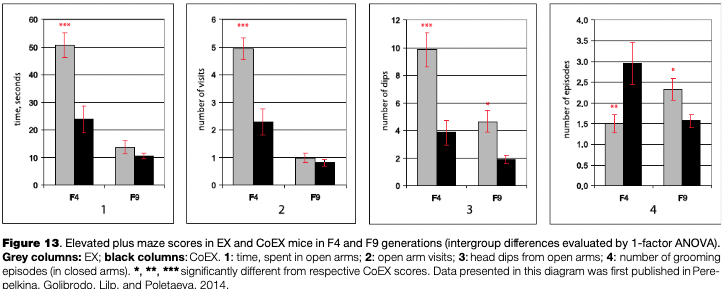
As it was mentioned above, the selection did not result in a large increase in the success of extrapolation task solutions during the initial selection generations, but a definite decrease in anxiety levels occurred. The anxiety level was estimated independently in the elevated plus-maze test (EPM).
Several scores of this test — time spent in the open maze arms, the number of visits into them, head dipping from open arms, and the numbers of grooming episodes — were significantly different between EX and CoEX mice groups (for generation F4, see Fig. 13). According to the generally accepted view, these differences indicate that the selected EX line expresses a decreased level of anxiety. So, at that stage of the selection experiment it was possible to conclude that while there was little selection success in cognitive trait values, the second trait which was selected for (low fear in extrapolation test) reduced markedly.
Despite similar selection criteria used throughout all selection generations, the data for the EPM test in F9 (and later generations, data for which are not presented) revealed a complicated pattern of differences between EX and CoEX mice in anxiety indices. Fig. 13, in which EPM scores for F4 and F9 are presented, shows this change rather clearly. At the same time, data for later generations (F9-F11) indicated the prevalence of EX mice performance in the “puzzle-box” solution and hyponeophagia tests.
The whole body of data on the coordinated expression (and/or mutual inhibitory influences) of cognitive abilities, reactions to novelty and anxiety, as well as the probable causes of sex differences in these indices, could not be discussed here. A plethora of experimental data obtained in comparative psychology and neurogenetics research demonstrate that anxiety is not the uniform state of animal CNS. It is claimed that this state can be evoked by different mechanisms and stimuli, according to the definite behavioral context (Johansen, 2013). This notion, not yet specified clearly in the literature, could find some confirmation in our data from anxiety levels during the selection of the EX strain.
Specifically, we postulate that the anxiety state, which in the EPM test is measured as the fear of the new open space (the animal avoids the open arms of the maze), and fear which the mouse experiences in the puzzle-box and which drives it to escape into the dark compartment, are not the identical states by their physiological (and maybe “instinctive”) origin. The latter state, which drives the animal to seek the dark part of the box, is closer to cautious behavior, while fear in the EPM looks more like real fear (which could induce “freezing” or “fleeing” reactions). We dare to suggest that if these two states are identical to one another, the pattern of differences between EX and CoEX mice would be of another sort. The same consideration is true when the EPM and hyponeophagia tests are compared — the EX mice have higher scores than CoEX mice in the reaction to novelty, in spite of a plausible anxiety state which is inevitable in the novel environment. It is also noteworthy that the pattern of differences in the EPM test for EX vs. CoEX mice does not coincide with their differences in reactions to novelty. In other words, the EX superiority in the test for novelty could not be predicted by data from the EPM test.
In our selection experiment, mice with high cognitive trait scores were bred and the scores of next generations were compared to unselected animals which possessed related genetic backgrounds. The changes in the extrapolation task success were minimal and unstable, while the selected line (EX) was proved to be different from control animals in other trait, which is considered indicative of a certain level of cognitive ability in animals. In the puzzle-box and hyponeophagia tests, mice of the selected line were significantly different from the control group, revealing the elevated novelty reaction and the better adaptive usage of the “object permanence” rule.
8. Conclusions
The phenomenological diversity of cognitive abilities in animals is evident due to numerous investigations in many species. The abundance of these studies necessitated the elaboration of a unified paradigm which will help to classify related experimental data obtained using different approaches. This will help to reliably evaluate animal cognitive abilities in species with different levels of organization. The subdivision of animal cognition phenomena into two main categories — the category of basal cognitive abilities and the category of complicated cognitive functions — could facilitate further comparative analyses of these phenomena, as their differences and common features could appear and be elucidated.
Following this subdivision, we analyzed the capacity of laboratory mice to solve the elementary reasoning task (as one of the basal cognitive abilities) in several laboratory genetic models. These models were: i) two pairs of wild vs. domesticated forms of brown rats and foxes; ii) mice with chromosomal rearrangements; iii) mice selected for large and small relative brain weight; and iv) mice selected for the high scores of reasoning task solution (extrapolation task).
It is intuitively clear why domesticated forms were less capable of solving the extrapolation task. The artificial selection in both species (Rattus norvegicus and Vulpes vulpes fulvus) was performed relatively recently. White rats were introduced into laboratory practice at the beginning of the 20th century, and silver foxes as the objects of fur farming were already known several years earlier. The artificial selection in these species aimed to create docile and tame animals which were not afraid of contact with humans. Both domesticated forms were maintained in cages, so there was no other selective force in action which could reject individuals with low adaptive intellectual capacities. Thus, domesticated rats (and foxes) who lived in cages and always had abundant food, for which they needed not to struggle, were shown to be less able to solve the elementary logic task. This basal cognitive behavior was significantly less developed in domesticated forms.
Mice of inbred strains, which were tested in our experiments, solved the extrapolation task in a proportion which evidenced their “random” performance. Indeed, one may suggest that animals which were able to solve this task reliably were present among the mice of inbred strains as well, although they were not numerous. The overall performance success (proportion of correct choices) in these groups was about 50%, indicating that a majority of inbred mice were not able to solve this elementary reasoning task. Animals which were capable of solving the extrapolation problem were more numerous among the genetically heterogeneous mouse population (created as the result of several inbred strains crossings). This fact served as the rationale to perform two selection experiments: the first for high and low relative brain weights, and the second for high scores of extrapolation task solution. The results of both selection programs demonstrated changes in mouse behavior in the successive generations, although in both experiments no stable increase in extrapolation ability occurred. The levels of success in the extrapolation task were not constant across selection generations, and notable sex differences emerged (at least in the selection for high extrapolation ability).
In an attempt to describe the general pattern of these results, one may conclude the following: i) the ability to solve the elementary logic task is by no means mono- or oligogenic; ii) it is also not a typical additive polygenic trait whereby the cumulative gene action selected for leads to a gradual increase in scores of the given quantitative trait — obviously, this trait is not determined by the additive action of “polygene” alleles; and iii) this means that the cognitive ability for elementary reasoning belongs to the category of traits, which are determined by a group of genes with non-additive effects. In these cases, artificial selection is always a slow process. The quantitative genetics rules state that weak selection gain is frequently the case for traits with non-additive gene action.
The selection process for large and small relative brain weight demonstrated that the large-brained mice displayed less propensities for fear and depressive behavior in laboratory tests, and that their extrapolation task scores were higher than those for smallbrained mice. The tendency for better behavior adaptation in the complicated test environment were also inherent for mice of the EX line (selected, although with low success) for high extrapolation scores. EX mice solved the “puzzle-box” cognitive task reliably better than controls and they demonstrated a positive attitude towards novel food in a novel environment (hyponeophagia test). These differences between the selected line and controls undoubtedly have a genetic component. It is an indication that genotype differences do participate in individual variability of the trait under consideration — the solution success of an elementary reasoning task, which illustrates a basal cognitive ability.
This conclusion is supported by our data which demonstrated a reliably higher extrapolation ability in mice with the fusion (robertsonian translocation) of chromosomes 8 and 17. The prevalence of correct choices in the extrapolation task experiments in mice-carriers of this mutation demonstrated that subtle (but efficient for behavior adaptation) genotype changes could be induced by the reorganization of chromosomes. Such reorganization, presumably the change in spatial pattern of chromosomes in the interphase nucleus which could influence the pattern of gene expression, resulted in the case of Rb (8,17) 1 lem in behavioral changes. Such changes have not (yet!) been achieved during more than ten generations of artificial selection for the same trait.
The usage of animal models to investigate cognitive abilities is aimed (in a majority of cases) at visualizing the deleterious effects of definite treatments or states. This is the well-established way to create the models used to study Alzheimer’s Disease and other pathological states. This line of experimental research has practical importance and generates a lot of new data for analysis. Attempts to increase cognitive abilities in animals are more rare in modern experimental practice, and they have been successful mainly when using knockout and knock-in modern technologies. The data described herein are from a rare category of studies in which the research was performed for animal genetic groups which show the increased cognitive capacities. The increase in cognitive abilities of laboratory mice could not be easily achieved by a direct selection process. This probably means that the additive variability of the respective genetic endowment of this complicated trait is either low or very low. It could signify that in spite of many generations of laboratory breeding (from the beginning of the 20th century), the overall fitness of laboratory mice is still rather high (as our cognitive trait under selection could not be improved quickly). This conclusion could be regarded as contradicting the prevalence of correct extrapolation task solutions in wild vs. domesticated rats and foxes. However, it is worth noting that the selection of rats for high extrapolation scores was not successful, either. We should also underline that extrapolation ability is still a unique example of basal cognitive abilities which was investigated in the special selection experiment. No other cognitive traits (i.e., spatial learning and memory) were selected for high and/or low scores of these traits. At the same time, despite the fact that genetic bases for basal cognitive traits look rather complicated, our data inspire moderate optimism in this respect.
In sum, the data presented show that by using laboratory mice (widely accepted as the model organism for studying genotype-behavior interactions) it was possible to demonstrate the role of genotype in determining the simplest forms of cognitive abilities.
References
Ammassari-Teule, M., & Caprioli, A. (1985). Spatial learning and memory, maze running strategies and cholinergic mechanisms in two inbred strains of mice. Behavioural brain research, 17(1), 9-16. doi: 10.1016/0166-4328(85)90003-8 Arp, J. M., ter Horst, J. P., Kanatsou,S., Fernandez, G., Joels, M.,
Krugers, H. J., & Oitzl, M. S. (2014). Mineralocorticoid receptors guide spatial and stimulus-response learning in mice. PLoS One, 9(1), e86236. doi: 10.1371/journal.pone.0086236 Bagotskaya,M.S., Smirnova, A. A., & Zorina, Z. A. (2012). Corvidae can understand logical structure in baited string-pulling tasks. Neuroscience and Behavioral Physiology, 42(1), 36-42.
Bakloushinskaya I. Y, Romanenko, S. A., Graphodatsky A.S.,Matveevsky, S. N., Lyapunova, E. A., & Kolomiets, O. L.. The role of chromosome rearrangements in the evolution of mole voles of the genus Ellobius (Rodentia, Mammalia). Russian journal of genetics, 46(9), 1143-1145. doi: 10.1134/S1022795410090346
Baranov,V.S. (1984). Mice with Robertsonian translocations in experimental biology and medicine. Genetica, 52(1), 23-32. doi: 10.1007/BF00121810
Becker,J.T., Walker,J.A., & Olton,D.S. (1980). Neuroanatomical bases of spatial memory. Brain Research, 200(2), 307-320. doi: 10.1016/0006-8993(80)90922-1
Ben-Abdallah, N.M., Fuss, J., Trusel, M., Galsworthy, M.J., Bob- sin, K., Colacicco, G., Deacon, R.M., Riva, M.A., Kellen- donk, C., Sprengel, R., Lipp, H-Р., Gass, P. (2011). The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Experimental Neurology, 227(1), 42-52. doi: 10.1016/j.expneurol.2010.09.008
BeritovJ.S. (1967). Neural mechanisms of higher vertebrate behavior. Translated and edited by W. T. Liberson. Boston: Little, Brown and Co.
Boake,C.R., Arnold, S. J., Breden,E, Meffert, L.M., Ritchie, M. G., Taylor, B. J., Wolf, J. B. & Moore, A. J. (2002). Genetic tools for studying adaptation and the evolution of behavior. The American Naturalist, 160(S6), S143-S159. doi: 10.1086/342902
Buresovä, O. (1980). Spatial memory and instrumental conditioning. Acta neurobiologiae experimentalis, 40(1), 51-65.
Buzsäki,G., Chen,L.S., 8c Gage,EH. (1990). Chapter Spatial organization of physiological activity in the hippocampal region: relevance to memory formation. Progress in brain research, 83, 257-268. doi: 10.1016/80079-6123(08)61255-8
Clinton, S.M., Stead, J. D., Miller, S., Watson, S. J., & Akil,H.. Developmental underpinnings of differences in rodent novelty seeking and emotional reactivity. European Journal of Neuroscience, 34(6), 994-1005. doi: 10.1 lll/j.1460-9568.2011.07811.x
Committee on Standardized Genetic Nomenclature for Mice. (1963). A revision of the standardized genetic nomenclature for mice. Journal of Heredity, 54,159-162.
Crabbe, J. C., Wahlsten,D., & Dudek, В. C. (1999). Genetics ofmouse behavior: interactions with laboratory environment. Science, 284(5420), 1670-1672. doi: 10.U26/science.284. 5420.1670
CrusiOjW.E., Schwegler,H., & Brust,I. (1993). Covariations between hippocampal mossy fibres and working and reference memory in spatial and non-spatial radial maze tasks in mice. European Journal ofNeuroscience, 5(10), 1413-1420. doi: 10.111 l/j.l460-9568.1993.tb00927.x
de Bruin, N., Mahieu,M., Patel, T, Willems, R., Lesage, A., & Megens,A. (2006). Performance of F2 B6xl29 hybrid mice in the Morris water maze, latent inhibition and prepulse inhibition paradigms: comparison with C57B1/6J and 129sv inbred mice. Behavioural brain research, 172(1), 122-134. doi: 10.1016/j.bbr.2006.05.002
Dragoi, G. & Tonegawa, S. (2014). Selection of preconfigured cell assemblies for representation of novel spatial experiences. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1635), 20120522. doi: 10.1098/rstb.2012.0522
DuclotjE, Jacquet,C., Gongora, C., & Maurice, T. (2010). Alteration of working memory but not in anxiety or stress response in рЗОО/CBP associated factor (PCAF) histone acetylase knockout mice bred on a C57BL/6 background. Neuroscience letters, 475(3), 179-183. doi: 10.1016/j.neulet.2010.03.077
Dulawa, S. C., & Hen, R. (2005). Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypo- phagia test. Neuroscience & Biobehavioral Reviews, 29(4), 771-783. doi: 10.1016/j.neubiorev.2005.03.017
Firsov,L.A. (2010). Ape’s behavior in natural habitat. Moscow: Krasand Publ.House, 2nd edition.
Firsov, L. A., & Chizhenkov,A.M. (2003). [The essays in physiological psychology]. Saint Petersburg: Aster-X Publ. House. (Russian).
Goh, J. J., & Manahan-Vaughan, D. (2013). Synaptic depression in the CAI region of freely behaving mice is highly dependent on afferent stimulation parameters. Frontiers in integrative neuroscience, 7: 1. doi: 10.3389/fnint.2013.00001
Goldowitz, D., Wahlsten,D., & Wimer, R.E. (1992). Techniques for the genetic analysis of brain and behavior: focus on the mouse (Vol. 8). Elsevier Science Ltd.
Grootendorst, J., Enthoven,L., Dalm,S., de Kloet,E.R., & OitzljM.S. (2004). Increased corticosterone secretion and early-onset of cognitive decline in female apolipoprotein E-knockout mice. Behavioural brain research, 148( 1), 167-177. doi: 10.1016/80166-4328(03)00188-8
Gropp, A., Winking, H., Redi, C., Capanna, E., Britton-Davidian, J., & Noack, G. (1982). Robertsonian karyotype variation in wild house mice from Rhaeto-Lombardia. Cytogenetic and Genome Research, 34(1-2), 67-77. doi: 10.1159/000131794
Hartley,T., Lever,C., Burgess,N., & O’Keefe,). (2014). Space in the brain: how the hippocampal formation supports spatial cognition. Philosophical Transactions of the Royal Society B: Biological Sciences, 369(1635), 20120510. doi: 10.1098/ rstb.2012.0510
Hasenöhrl, R. U., Weth, K, & Huston, J. P. (1999). Intraventricular infusion of the histamine H(l) receptor antagonist chlorpheniramine improves maze performance and has anxio- lytic-like effects in aged hybrid Fischer 344xBrown Norway rats. Experimental Brain Research, 128(4), 435-440. doi: 10.1007/S002210050866
Henderson, N.D. (1973). Brain weight changes resulting from enriched rearing conditions: a diallei analysis. Developmental psychobiology, 6(4), 367-376. doi: 10.1002/dev.420060410 Herrera, V. L., Pasion, K.A., Tan,G.A., Moran, A. M., & Ruiz-
Opazo,N. (2013). Sex-Specific Effects on Spatial Learning and Memory, and Sex-Independent Effects on Blood Pressure of a <3.3 Mbp Rat Chromosome 2 QTL Region in Dahl Salt-Sensitive Rats. PloS one, 8(7), e67673. doi: 10.1371/ journal.pone.0067673
Hussaini, S. A., Kempadoo, K. A., Thuault, S. J., Siegelbaum, S. A., & Kandel, E. R. (2011). Increased size and stability of GAI and GA3 place fields in HCN1 knockout mice. Neuron, 72(4), 643-653. doi: 10.1016/j.neuron.20U.09.007
Johansen, J. P. (2013). Neuroscience: Anxiety is the sum of its parts. Nature,496(7444), 174-175. doi: 10.1038/naturel2087
Koehler, O. (1956). Thinking without words. In Proceedings of the 14th International Congress of Zoology, Copenhagen (pp. 75-88).
Kohler, W. (1921). Intelligenzprüfung an Menschenaffen [The mentality of apes], Berlin: Springer, doi: 10.1007/978-3- 642-47574-0
Korsten, P., Mueller, J. C., Hermannstaedter, C., Bouwman, K. D., Dingemanse, J. N., Piet, N. S. J., Liedvogel, J. D. M., Matthy- sen, E., Van Oers, K., Van Overveld, T, Quinn, J. L., Patrick, S. C., Sheldon, B. N. C., Tinbergen, J. M., & Kem- penaers, B. (2010). Association between DRD4 gene polymorphism and personality variation in great tits: a test across four wild populations. Molecular Ecology, 19(4), 832-843. doi: 10.11U/j.l365-294X.2009.04518.x
Krushinsky,L.V. (1990). Experimental studies of elementary reasoning: Evolutionary, physiological, and genetic aspects of behavior. New Delhi, Oxonian Press.
Krushinsky,L.V. (2009). [Biological basis of reasoning ability. Evolutionary, physiological, and genetic aspects]. 3rd edition (l8t and 2nd - Moscow University Publ.House, 1977,1986). Moscow: URSS Publ. House, Moscow. (Russian).
Krushinskii, L. V, Dyban,A.P., Baranov, V.S., Poletaeva, I. L, & Romanova, L. G. (1982). [Features of higher nervous activity in mice with Robertsonian chromosomal translocations], Zhurnal vysshei nervnoi deiatelnosti imeni I. P. Pavlova, 32(3), 446. (Russian).
Krushinskii, L., Dyban,A.P., Baranov, V. 8., Poletaeva, I. L, Romanova, L. G., Foreit,!., & Gregorova, 8. (1982). [Investigation of the extrapolation ability in laboratory mice with partial trisomy of autosomes], Doklady Akademii nauk SSSR, 260(6), 1497-1499. (Russian).
Kruska, D. (1974). [Comparative quantitative study on brains of wild and laboratory rats. I. Comparison of volume of total brain and classical brain parts ] Journal fur Hirn- forschung 16(6), 469-483.
Kruska, D. C. (2005). On the evolutionary significance of encepha- lization in some eutherian mammals: effects of adaptive radiation, domestication, and feralization. Brain, behavior and evolution, 65(2), 73. doi: 10.1159/000082979
Kuptsov,P.A., Pleskacheva,M.G., & Anokhin, К. V (2011). [Inhomogeneous hippocampal activation along the rostrocau- dal axis in mice after exploration of novel environment], Zhurnal vysshei nervnoi deiatelnosti imeni IP Pavlova, 62(1), 43-55. (Russian).
Kuptsov,P. A., Pleskacheva,M. G., Voronkov, D. N., Lipp,K.P., & Anokhin, К. V. (2006). Features of the expression of the c-Fos gene along the rostrocaudal axis of the hippocampus in common voles after rapid training to solve a spatial task. Neuroscience and behavioral physiology, 36(4), 341-350. doi: 10.1007/sll055-006-0023-y
Ladygina-Kots, N. N. (2002). Infant Chimpanzee and Human Child: A Classic 1935 Comparative Study of Ape Emotions and Intelligence. Oxford University Press.
Laeremans, A., Sabanov, V, Ahmed, T, Nys,J., Van de Pias, B., Vinken, K., Woolley, D. G., Gantois, I., D’Hooge, R., Arck- ens,L., & Balschun, D. (2014). Distinct and simultaneously active plasticity mechanisms in mouse hippocampus during different phases of Morris water maze training. Brain Structure and Function, 1-18. doi: 10.1007/ S00429-014-0722-z
Leitinger, B., Poletaeva, I. L, Wolfer, D. P, & Lipp, H. P. (1994). Swimming navigation, open-field activity, and extrapolation behavior of two inbred mouse strains with Robertsonian translocation of chromosomes 8 and 17. Behavior genetics, 24(3), 273-284. doi: 10.1007/BF01067194
Lerch, J. P., YiUjA.P., Martinez-Canabal, A., Pekar,T., Boh- bot, V. D., Frankland, P. W, Henkelman, R. M., Josselyn, S. A., & Sled, J. G. (2011). Maze training in mice induces MRI- detectable brain shape changes specific to the type of learning. Neuroimage, 54(3), 2086-2095. doi: 10.1016/j.neuro image.2010.09.086
Lipp, H. P, Collins, R. L., Hausheer-Zarmakupi, Z., Leisinger- Trigona, M. C.,Crusio, W. E.,Nosten-Bertrand, M.,Signore, R, Schwegler, H., & Wolfer, D. P. (1996). Paw preference and intra-/infrapyramidal mossy fibers in the hippocampus of the mouse. Behavior genetics, 26(4), 379-390. doi: 10.1007/ BF02359482
Lipp, H. P., Schwegler, H., Crusio, W.E., Wolfer, D. P, Leisinger- Trigona,M.C., HeimrichjB., & Driscoll,P. (1989). Using genetically-defined rodent strains for the identification of hippocampal traits relevant for two-way avoidance behavior: a non-invasive approach. Experientia, 45(9), 845-859. doi: 10.1007/BF01954059
Markina,N.V., Salimov,R.M., & Poletaeva,LI. (2001). Behavioral screening of two mouse lines selected for different brain weight. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 25(5), 1083-1109. doi: 10.1016/ 80278-5846(01100169-5
Matsuo, N., Takao,K., Nakanishi, K., Yamasaki, N., Tanda,K., & Miyakawa,T. (2010). Behavioral profiles of three C57BL/6 substrains. Frontiers in behavioral neuroscience, 4. doi: 10.3389/fnbeh.2010.00029
Meck,W.H., Church,R.M., & Olton,D.S. (1984/2013). Hippocampus, time, and memory. Behavioral Neuroscence. 127(5), 655-668. doi: 10.1037/a0034188
Miyoshi, E., Wietzikoski, E. C., Bortolanza,M., Boschen, S. L., CanteraSjN.S., Izquierdo, I., & Da Cunha,C. (2012). Both the dorsal hippocampus and the dorsolateral striatum are needed for rat navigation in the Morris water maze. Behavioural brain research, 226(1), 171-178. doi: 10.1016/j. bbr.2011.09.011
Morris, R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods, 11(1), 47-60. doi: 10.1016/0165-0270(84)90007-4
Morris, R. G. (1981). Spatial localization does not require the presence of local cues. Learning and Motivation. 12, 239—260. doi: 10.1016/0023-9690(81)90020-5
Mueller, J. C., Korsten,P., Hermannstaedter,C., Feulner,T., Dinge- manse,N.S.J., Matthysen,E., Van Oers,K, Van Overveld, T., Patrick, S.C., Quinn, J. L., Riemenschneider, M., Tinbergen, J. M., Kempenaers,B. (2013). Haplotype structure, adaptive history and associations with exploratory behaviour of the DRD4 gene region in four great tit (Parus major) populations. Molecular ecology, 22(10), 2797-2809. doi: 10.1111/mec.l2282
Nguyen, P.V, Abel, T., Kandel, E.R., Bourtchouladze, R. (2000). Strain-dependent differences in LTP and hippocampusdependent memory in inbred mice. Learning & Memory, 7(3), 170-179. doi: 10.1101/lm.7.3.170
O’Keefe,)., & Nadel,L. (1978). The hippocampus as a cognitive map. Clarendon Press, Oxford.
Olton,D.S. (1972). Discrimination reversal performance after hippocampal lesions: an enduring failure of reinforcement and non-reinforcement to direct behavior. Physiology & behavior, 9(3), 353-356. doi: 10.1016/0031- 9384(72)90158-8
Olton,D.S. (1977). Spatial memory. Scientific American, 236, 82-98. doi: 10.1038/scientificamerican0677-82
Olton,D.S. (1992). Tolman's cognitive analyses: predecessors of current approaches in psychology. Journal of Experimental Psychology. General, 121, 427-428. doi: 10.1016/0031-9384(72)90158-8
Olton, D. S., 8r Samuelson, R. J. (1976). Remembrance of places passed: Spatial memory in rats. Journal of Experimental Psychology: Animal Behavior Processes, 2(2), 97. doi: 10.1037/0097-7403.2.2.97
Olton, D. S., Markowska, A. L., Pang, K., Golski, S., Voytko, M. L., & Gorman, L. K. (1992). Comparative cognition and assessment of cognitive processes in animals. Behavioural pharmacology, 3(4), 307-318. doi: 10.1097/00008877-199208000-00006
Pang,K., Williams,M. J., Egeth,H., & Olton,D.S. (1993). Nucleus basalis magnocellularis and attention: effects of muscimol infusions. Behavioral neuroscience, 107(6), 1031-1038. doi: 10.1037/0735-7044,107.6.1031
Paratore, S., Alessi, E., Coffa, S., Torrisi,A., Mastrobuono, E, & Cavallaro, S. (2006). Early genomics of learning and memory: a review. Genes, Brain and Behavior, 5(3), 209-221. doi: 10.1111/І.1601-183Х.2005.00159.Х
Patil, S. S., Schlick, E, Höger, H., & Lubec, G. (2010). Involvement of individual hippocampal signaling protein levels in spatial memory formation is strain-dependent. Amino Acids, 39(1), 75-87. doi: 10.1007/s00726-009-0379-8
PazhetnoVjV.S. (1990). [The brown bear]. Moscow: Agropromiz- dat. (Russian).
Peirce, J. L., Chesler, E. J., Williams, R.W. & Lu, L. (2003). Genetic architecture of the mouse hippocampus: Identification of gene loci with selective regional effects. Genes, Brain and Behavior, 2,238-252. doi:10.1034/j.l601-183X.2003.00030.x
Perepelkina, О. V, Golibrodo,V.A., Lilp,I.G., & Poletaeva, 1.1. (2014). Selection of laboratory mice for the high scores of logic task solutions: The correlated changes in behavior. Advances in Bioscience and Biotechnology, 5, 294-300. doi: 10.4236/abb.2014.54036
Perepelkina, O.V, Markina, N.V, Golibrodo,V. A., Lil'p, I. G., 8k Poletaeva, 1.1. (2011). [Selection of mice for high level of extrapolation capacity with cobcommitant low anxiety level], Zhurnal vysshei nervnoi deiatelnosti imeni IP Pavlova, 61(6), 742-749. (Russian).
Pleskacheva, M. G., & Zorina, Z. A. (2012). Solution of Revecz- Krushinskii test by animals of different taxonomic groups. Journal of Evolutionary Biochemistry and Physiology, 48(5-6), 548-567. doi: 10.1134/S002209301205009X
Pleskacheva, M. G., Zorina, Z. A., Nikolenko, D. L., Wolfer, D. P, Kostyna,Z. A., 8k Lipp, H. P. (2002). [Behavioral impairments in Morris water maze in rats selectively bred for audiogenic seizure susceptibility (Krushinsky-Molodkina strain)]. Zhurnal vysshei nervnot deiatelnosti imeni IP Pavlova, 52(3), 356-365. (Russian).
Pleskacheva, M. G., Wolfer, D. P, Kupriyanova, I. E, Nikolenko, D.L., Scheffrahn, H., DelTOmo, G., & Lipp, H. P. (2000). Hippocampal mossy fibers and swimming navigation learning in two vole species occupying different habitats. Hippocampus, 10(1), 17-30. doi: 10.1002/ (SIGI) 1098-1063(2000) 10:l< 17::AID-HIPO2>3.Q.CO:2-O
Poletaeva, I. L, & Romanova, L. G. (2013). [Chromosomal mutations and mouse ability to extrapolate the direction of stimulus movement]. In: Poletaeva 1.1., Zorina Z.A.(eds)). The development of behavior: its normal and abnormal aspects. To the 100 anniversary ofL. V. Krushinsky. Moscow: LRS Publ. House, 217-238. (Russian).
Poletaeva, I. L, & Zorina, Z. A. (eds.) (2013). The development of behavior: its normal and abnormal aspects. To the 100 anniversary ofL. V. Krushinsky. LRS Publ. House. (Russian).
Poletaeva, I. L, Popova, N.V., & Romanova, L. G. (1993). Genetic aspects of animal reasoning. Behavior Genetics, 23,467-475. doi:10.1007/BF01067982
Popova, N. V., 8k Poletaeva, 1.1. (1983). [Ability to solve extrapolation problems in mice selected for different brain weights], Zhurnal vysshei nervnoi deiatelnosti imeni IP Pavlova, 33, 370-372. (Russian).
Popova, N.V., Kessarev, V. S., Poletaeva, I. I., & Romanova, L. G. (1983). [Cortical cytoarchitectonics in mice, selected for large and small brain weight], Zhurnal vysshei nervnoi deiatelnosti imeni IP Pavlova, 33, 576-582. (Russian).
Rehkämper, G., Frahm, H. D., 8k Cnotka, J. (2008). Mosaic evolution and adaptive brain component alteration under domestication seen on the background of evolutionary theory. Brain, behavior and evolution, 71(2), 115-126. doi: 10.1159/000111458
Rensch, B. (1956). Increase of learning capability with increase of brain-size. American Naturalist, 81-95. doi: 10.1086/281911
Reznikova, Z. (2007). Animal intelligence. From individual to social cognition. Cambridge University Press.
Ruiz-Opazo, N., & Tonkiss, J. (2006). Genome-wide scan for quantitative trait loci influencing spatial navigation and social recognition memory in Dahl rats. Physiological genomics, 26(2), 145-151. doi: 10.1152/phy siolgenomics.OOO 19.2006
Sago, H., Carlson, E. J., Smith, D. J., Kilbridge, J., Rubin, E.M., Mobley, W.C., Epstein,C. J., & Huang,T.T. (1998). TslCje, a partial trisomy 16 mouse model for Down syndrome, exhibits learning and behavioral abnormalities. Proceedings of the National Academy of Sciences, 95(11), 6256-6261. doi: 10.1073/pnas.95.11.6256
Sara,S.)., Devauges,V., Liegen,A., & Blizard,D.A. (1994). The Maudsley rat strains as a probe to investigate noradrenergic-cholinergic interaction in cognitive function. Journal of Physiology-Paris, 88(6), 337-345. doi: 10.1016/0928-4257(94)90026-4
Sarnyai,Z., Sibille, E. L., Pavlides,C., Fenster, R. J., McEwen, B.S., & T6th,M. (2000). Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin 1A receptors. Proceedings of the National Academy of Sciences, 97(26), 14731-14736. doi: 10.1073/pnas.97.26.14731
Schwegler, H., Crusio,W.E., & Brust, I. (1990). Hippocampal mossy fibers and radial-maze learning in the mouse: a correlation with spatial working memory but not with поп-spatial reference memory. Neuroscience, 34(2), 293-298. doi: 10.1016/0306-4522(90)90139-11
Schwegler,H., Crusio,W.E., Lipp,H.R, Brust,!., & Mueller,G.G. (1991). Early postnatal hyperthyroidism alters hippocampal circuitry and improves radial-maze learning in adult mice. The Journal of neuroscience, 11(7), 2102-2106.
Shumaker,R.W., Walkup,K.R., & Beck,В.B. (2011). Animal tool behavior: the use and manufacture of tools by animals. JHU Press.
Steinberger, D., Reynolds, D. S., Ferris, P., Lincoln, R., Datta,S., Stanley,)., Paterson,A., Dawson,G.R., & Flint,). (2003). Genetic mapping of variation in spatial learning in the mouse. The Journal of neuroscience, 23(6), 2426-2433.
Sultana,R., Ameno,K., )amal,M., Miki,T., Tanaka,N., Ono,)., Kinoshita,H. & Nakamura,Y. (2013). Low-dose nicotine facilitates spatial memory in ApoE-knockout mice in the radial arm maze. Neurological Sciences, 34(6), 891-897. doi: 10.1007/S10072-012-1149-Z
Sunyer,B., Patil, S., Frischer, C., Hoeger, H., & Lubec, G. (2008). Strain-dependent effects of cognitive enhancers in the mouse. Amino Acids, 34(3), 485-495. doi: 10.1007/ S00726-007-0511-6
leather, L. A., Packard, M.G., Smith, D. E., Ellis-Behnke, R. G., & Bazan, N. G. (2005). Differential induction of c-)un and Fos- like proteins in rat hippocampus and dorsal striatum after training in two water maze tasks. Neurobiology of learning and memory, 84(2), 75-84. doi: 10.1016/j.nlm.2005.03.006
Terunuma,M., Revilla-Sanchez, R., Quadros, I. M., Deng, Q., Deeb, T. Z., Lumb,M., Sicinski,P., Haydon, P. G., Panga- IoSjM.N., & MosSjSJ. (2014). Postsynaptic GABAB Receptor Activity Regulates Excitatory Neuronal Architecture and Spatial Memory. The Journal of Neuroscience, 34(3), 804-816. doi: 10.1523/TNEUROSCL3320-13.2Q13
Tolman, E. G. (1932). Purposive behavior in animals and men. London: Appleton-Gentury.
Tolman, E. C. (1948). Cognitive maps in rats and men. Psychological review, 55(4), 189-208. doi: 10.1037/h0061626
Trut,L.N. (1999). Early Canid Domestication: The Farm-Fox Experiment: Foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay between behavioral genetics and development. American Scientist, 160-169. doi: 10.1511/1999.2.160
Upchurch,M., & WehnerJ.M. (1989). Inheritance of spatial learning ability in inbred mice: a classical genetic analysis. Behavioral neuroscience, 103(6), 1251—1258. doi: 10.1037/0735-7044,103.6.1251
V6ikar,V., Pohis, A., Vasar,E., & Rauvala, H. (2005). Long-term individual housing in C57BL/6) and DBA/2 mice: assessment of behavioral consequences. Genes, Brain and Behavior, 4(4), 240-252. doi: 10.1111/j.l601-183X.2004.00106.x
Wahlsten,D. (2011). Mouse behavioral testing. How to use mice in behavioral neuroscience. Elsevier, Academic Press.
Wahlsten,D., Cooper,8.E, & Crabbe,).C. (2005). Different rankings of inbred mouse strains on the Morris maze and a refined 4-arm water escape task. Behavioural brain research, 165(1), 36-51. doi: 10.1016/j.bbr.2005.06.047
Wang, В., Hu, Q., Hearn, M.G., Shimizu, K., Ware, С. B., Ligg- ittjD. H., )in,L.W, Cool, В. H., Storm, D.R., & Martin, G. M. Isoform-specific knockout of FE65 leads to impaired learning and memory. Journal of neuroscience research, 75(1), 12-24. DOI: 10.1002/jnr. 10834
Wasserman, E. A., & Zentall,T.S. (eds). Comparative cognition. Experimental exploration of animal intelligence. N.Y: Oxford University press, 2006.
WehnerJ.M., Radcliffe,R.A., Rosmann,S.T, Christensen,S.C., Rasmussen, D. L., Fulker,D.W, & Wiles,M. (1997). Quantitative trait locus analysis of contextual fear conditioning in mice. Nature genetics, 17(3), 331-334. doi: 10.1038/ ngl 197-331
Wenk, G., Hughey, D., Boundy, V., Kim, A., Walker, L., & Olton, D. (1987). Neurotransmitters and memory: role of cholinergic, serotonergic, and noradrenergic systems. Behavioral neuroscience, 101(3), 325-332. doi: 10.1037/0735-7044.101.3.325
Williams, R.W, & Mulligan, M. K. (2011). Genetic and molecular network analysis of behavior. International review of neurobiology, 104, 135-157. doi: 10.1016/ B978-0-12-398323-7.00006-9
Williams,R.W. (n.d.). Measuring brain weight. Retrieved from http://www.nervenet.org/iscope/mbl 10.html
Wincott, С. M., Abera, S., Vunck, S. A., Choi,Y, Titcombe, R. E, Antoine, S. O., Tukey,D.S., Devito, L. M., Hofmann, E, Hoeffer,C. A., & Ziff, E. B. (2014). cGMP-dependent protein kinase type II knockout mice exhibit working memory impairments, decreased repetitive behavior, and increased anxiety-like traits. Neurobiology of learning and memory, 114, 32-39. doi: 10.1016/i.nlm.2014.04.007
Yadav, R., Hillman, B. G., Gupta, S. C., Suryavanshi, P., Bhatt J. M., Pavuluri,R., Stairs,DJ., & Dravid,S.M. (2013). Deletion of glutamate delta-1 receptor in mouse leads to enhanced working memory and deficit in fear conditioning. PLoS One, 8(4):e60785. doi: 10.1371/journal.pone.0060785
Yoshida, M., Goto, K., & Watanabe,S. (2001). Task-dependent strain difference of spatial learning in C57BL/6N and BALB/c mice. Physiology & behavior, 73(1), 37-42. doi: 10.1016/S0031-9384(0D00419-X
Zorina, Z. A., & Obozova,T. A. (2012). New data on the brain and cognitive abilities of birds. Biology Bulletin, 39(7), 601-617. doi: 10.1134/S1062359012070126
Zorina, Z. A. & Poletaeva, 1.1. (2001/2011). [Zoopsychology: elementary thinking in animals], Moscow: Aspect Press. (Russian).
Zorina, Z. A., & Smirnova, A. A. (2013). [The history of experimental studies of cognitive abilities and the impact of L. V. Krushinsky in the modern concepts of elementary reasoning], In: The development of behavior: its normal and abnormal aspects. To the 100 anniversary ofL. V. Krushinsky (pp. 36-38). Moscow: LRS Publ. (Russian).





